California e-Pedigree legislation requires that 50% of pharmaceuticals shipped to the state starting in 2015 be tracked and traced down to the individual bottle level. ESS Technologies (www.esstechnologies.com), a packaging line manufacturer, has partnered with Xyntek Inc. (www.xyntekinc.com), a serialization, track & trace and machine vision solution provider, to provide a robotic case erector/loader with integrated track and trace capability. This system allows pharmaceutical and medical device manufacturers to automate the case packing process and provide unit-level serialization, traceability and authentication for all of the products being case packed.
Securing the pharmaceutical supply chain
e-Pedigree legislation has been driven by the complexity of the drug distribution supply chain, which makes it difficult to prevent diversion and counterfeiting. The e-Pedigree consists of an electronic record of each transaction resulting in a change in ownership of the drug from the initial sale by the manufacturer through wholesalers and distributors and pharmacies until final sale to the consumer. The e-Pedigree must be compatible through all stages of the supply chain, which requires collaboration.
Even without e-Pedigree laws, drug manufacturers are interested in tracing pharmaceuticals in order to stop counterfeiting, minimize production and distribution of unsafe or poor quality products and implement a granular tracing system that helps reduce the magnitude of recalls. Even though all regions have not yet enacted e-Pedigree legislation, manufacturers exporting into regulated markets must adapt their packaging to conform even when the product is produced in jurisdictions that have not yet imposed traceability requirements.
Pharmaceutical manufacturers are addressing these challenges by implementing serialization solutions that affix a unique and traceable serial number on every package, bundle, case and pallet. This serial number is read many times as the product moves from manufacturer to consumer and each time an entry is made in a database to document the chain of custody during the entire product cycle.
Challenge of addressing manual case packing
The challenge is particularly great for the many lines around the world that are not fully automated. Typically these lines use automated filling and labeling and manual case and pallet packing and operate at relatively low speeds in the area of three to five cases per minute each containing six, 12 or 24 individual packages. While some companies have considered using their existing workforce to manually track and trace the packages, there are concerns that human operators cannot provide 100% assurance of an accurate pedigree. Manual operations are inherently vulnerable to untraceable errors, making it difficult or impossible to comply with e-Pedigree regulations.
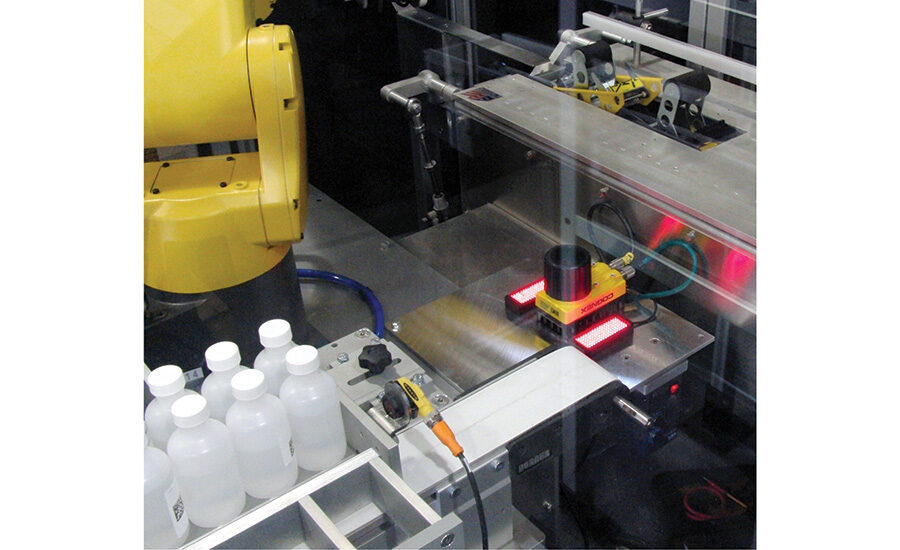
The ESS/Xyntek approach addresses this challenge by using a multi-axis robot with special end of arm tooling (EOAT) to package the product while providing unit-level serialization and track and trace technology for all of the products being case packed. When product is loaded into the case it’s important to determine which particular bottles are going into each case. The serial number on the labels of the bottles cannot be read at this point because the bottles are bunched togetherto be put into the case. However, the dummy codes on the bottom of the bottle can easily be read.
After filling and labeling but prior to packing the bottles in cases, the Xyntek-Antares Bottle Tracking System, which utilizes four Cognex (cognex.com) InSight 5603 vision systems, images all sides of the bottles in order to read the 2D DataMatrix code on the label regardless of its position while a single In-Sight 5603 vision system reads the dummy barcodes on the bottom of the bottles. The serial number on the label and the dummy code on the bottom of each bottle are both entered into a database so either one can easily be determined based on the other.
Why choose a vision system?
The traditional approach to pharmaceutical serialization uses industrial cameras multiplexed to an industrial personal computer. These systems can be expensive to maintain and validate. Xyntek selected the high-resolution, Cognex In-Sight 5603 vision system because of its powerful, high-speed processer and the fact that it operates independent of a PC for increased stability with less frequent updates and maintenance. Also, a single In-Sight 5603 can consistently read all 12 bottles within the time requirement.
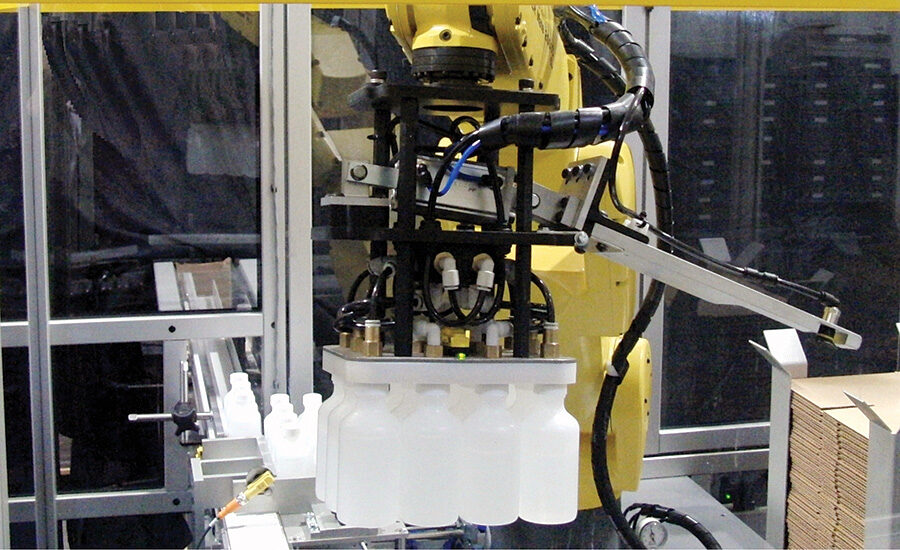
The bottles come into the robotic cell on a conveyor and a servo device forms them into a pattern based on the type of container, for example, a four by three pattern for a 12-pack. The robot then picks up all 12 bottles and loads them into the case. Vacuum sensors on the EOAT check for the presence of each bottle and if a bottle is missing it will set off an error signal. Vacuum cups on the EOAT pick up a case blank from a magazine. The robot uses either regular slotted container cases that are normally used in manual operations or wraparound cases that are common in automated processes.
100% bottle to case aggregation
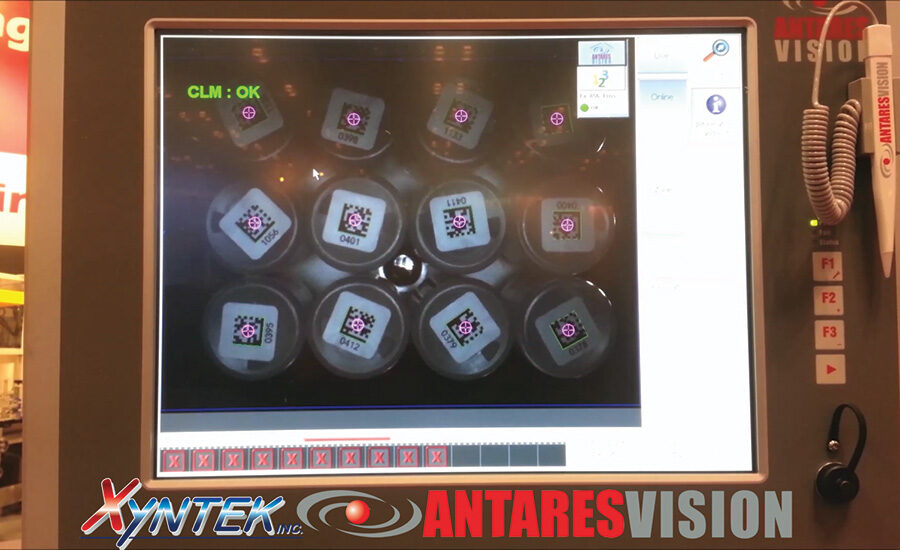
As the robot moves the bottles towards the case, it passes them over an In-Sight 5603 vision system that reads the dummy codes on the bottom of the bottles. These dummy codes have already been aggregated to the serial number of each bottle as recorded on the label. Reading the dummy code makes it possible to identify the unique serial number of the bottle. This aggregation enables 100% verification of the bottles as they are packed into the case. The robot loads the bottles into the case and then folds the minor and major flaps and seals the container with tape. The Xyntek-Antares Tracking System software confirms the unique serial numbers based on the pack pattern and prints a case label to complete the bottle to case aggregation. If the operation fails because a bottle is missing in the EOAT or because a label could not be read, the line stops.
The ESS/Xyntek solution provides a cost-effective and easy-to-implement way to upgrade existing lines to meet e-Pedigree regulations while maintaining existing machine speed and quality. The system provides an automated alternative to manual case packing processes while handling up to five cases per minute. It enables pharmaceutical and medical device manufacturers to automate the case packing process and provide unit serialization and track and trace technology for all of the products being case packed. Cognex vision systems play a critical role by providing a compact, fast, and accurate method of reading and verifying the quality of 1-D and 2-D barcodes.
To see a video of this technology in action, go to packagingstrategies.com/Cognexvideo